


![]()
ONLINE

Magnetecs’
Mission
Editors’ Note
Josh Shachar is an inventor and a mathematician with experience in the fields of advanced medical technologies and military systems. He has over 160 currently active patents and applications to his name. After military service in the Israel Defense Force, Shachar went to Paris where he studied philosophy and mathematics at the Sorbonne University. He returned to Israel where he graduated magna cum laude with bachelor’s and master’s degrees in the philosophy of science and mathematics from the University of Haifa, Israel. He then came to the United States to continue his studies as a Fulbright Scholar while attending UCLA and USC. Shachar has been involved in developing advanced technologies for the United States Department of Defense for over 20 years. He has held many executive management positions at high-technology companies dealing directly with the United States Department of Defense. He is the principal owner and founder of several other high-technology companies including ThermoCouple America LLC, EDEL Engineering Development Corp., and Engineered Magnetics, Inc., and founder and CTO of Pharmaco-Kinesis Corporation.
In 1999, after theoretical and practical studies and the initial patent applications, Shachar assembled the core Magnetecs engineering group to develop and commercialize the CGCI™ system and bring the benefits of CGCI™ technology to patients around the world. He has been leading the CGCI™ project from that point forward, and Magnetecs Corporation has been his primary focus since its founding in 2003.
Company Brief
Magnetecs Corporation (magnetecs.com) designs and manufactures a unique and highly efficient robotic catheterization control system, known as the CGCI System (for Catheter Guidance Control and Imaging), for minimally invasive surgical procedures, initially for the treatment of heart arrhythmia with additional applications in neurology to reduce the risk of aneurysms, and in other fields. The company is also developing advanced MOSFET (“metal-oxide semiconductor field-effect transistor”) sensor technology, which has the potential to enhance and transform the use of catheters and additional devices.
What is the focus for Magnetecs and would you highlight your solutions?
It’s the CGCI robotic navigation platform, in serving as the standard of care initially in electrophysiology, and in several additional fields of use such as aneurysm, renal denervation, neurology, and vasculature. The CGCI uses benign magnetic energy to remotely control tethered devices such as catheters and related tools using an intuitive control system that provides agile, real-time manipulation of such tools within the target anatomy with unmatched freedom of movement and control.
Advanced MOSFET sensor technology – which has the potential to fundamentally change the usage of catheters, pacing leads, and other devices – is another focus for us. The MOSFET sensor technology will provide a means of detecting faint bio-potential at a threshold an order of magnitude lower than the capability of existing devices.
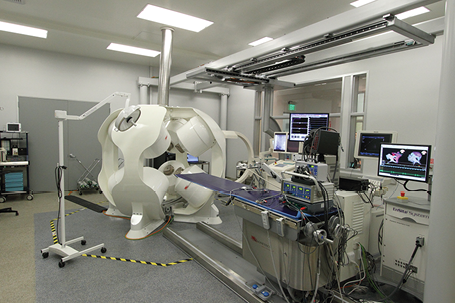
Magnetecs’ CGCI™ System
What makes Magnetecs’ global technology unique?
Magnetecs CGCI has the unique ability to provide an unrivaled degree of fine control, safety, efficacy, and repeatability of procedures using remote navigation. Through the use of our Catheter Guidance, Control, and Imaging system, the near-constant use of X-ray standard to existing procedures is greatly attenuated to a few minutes of exposure per hour.
The force delivered by the CGCI to the catheter tip is sufficient for firm contact with the target tissues during mapping and ablation, but does not cause puncture damage to the vasculature. Further safety features include haptic response to anatomical boundaries that allow the surgeon to feel the walls of the target site as a rigid force, which prevents attempts in steering of the catheter outside the safe working region of the location of operation.
Additionally, the CGCI system makes possible the performance of surgical procedures remotely, allowing a skilled physician using the control interface to conduct operations in another hospital across the globe.
What kind of impact will Magnetecs have on the proficiency of physicians and on the efficiency of hospitals and clinics?
The robotic system CGCI’s first indication is in the field of electrophysiology, where complex arrhythmia procedures are lengthy (three to five hours), with continuous exposure to X-radiation, and with indices of success that are limited. Using the CGCI platform, the physician’s dexterity is reduced to a machine language, and the operation mimics the ease of control of a video game. The accuracy and safety of the system enables quick and accurate access to a complex anatomy, while delivering the therapeutic procedure with limited exposure to X-radiation and substantial improvements on the patient outcome.
How do you focus your efforts in leading Magnetecs?
The development of the CGCI system went through an animal study phase led by Dr. Eli Gang, M.D. and his peers, whereby the software and clinical validation were established. Subsequently the human-scale machine was built and installed in La Paz Hospital (Madrid), and in Yonsei University Hospital in Seoul.
Human clinical trials are currently in progress led by Dr. Jose Merino at La Paz, and the results to date have demonstrated the versatility and unique advantage of CGCI’s navigation and control abilities.
The phase of development culminated in obtaining Magnetecs ISO13485 and its CE Mark for commercial use in Europe. Currently, the company is pursuing its FDA regulatory approval, which we expect to obtain within the year.
Magnetecs’ strategy has always been one of clinical validation of the technology to establish a solid foundation for the platform in its commercial phase.
What are your key priorities for Magnetecs?
Magnetecs CGCI has the potential to become a new standard of care for treatment of arrhythmias and other minimally invasive surgical procedures. Our goal is to establish the clinical validation of the technology through multi-centered, randomized trials at our installations in Madrid and Seoul, and at our upcoming site at Na Homolce Hospital in Prague. With this research and the support of key opinion leaders in the electrophysiology community – such as Dr. Vivek Reddy and Dr. Petr Neuzil – and the approval from the U.S. FDA, we envision that the CGCI will become familiar and standard equipment in electrophysiology labs at all centers of excellence.
In parallel, the development of our MOSFET sensor technology will engender a new class of sensory devices for electrophysiological studies, providing a level of detail never before seen in bio-potential signals, which will improve diagnostic results and pave the way to new procedures and tools utilizing these capabilities.•